Commercialization of medical artificial intelligence technologies: challenges and opportunities
Bajwa, J., Munir, U., Nori, A. & Williams, B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Health. J. 8, e188–e194 (2021).
Lareyre, F. & Raffort, J. Artificial intelligence in vascular diseases: from clinical practice to medical research and education. Angiology (2025).
Song, P. et al. The global and regional prevalence of abdominal aortic aneurysms: a systematic review and modeling analysis. Ann. Surg. 277, 912–919 (2023).
Google Scholar
Eid, M. A. et al. The global burden of peripheral artery disease. J. Vasc. Surg. 77, 1119–1126.e1 (2023).
Google Scholar
Sinha, K., Berczeli, M., Lumsden, A. B. & Roy, T. L. Imaging: new frontiers in vascular training. Methodist Debakey Cardiovasc. J. 18, 39–48 (2022).
Google Scholar
Zaidan, E. & Ibrahim, I. A. AI governance in a complex and rapidly changing regulatory landscape: a global perspective. Humanit. Soc. Sci. Commun. 11, 1121 (2024).
Mastracci, T. M., Anand, S. S. & Aday, A. W. Peripheral artery disease: a high-risk yet understudied, underdiagnosed, and undertreated condition-a call to action. Can. J. Cardiol. 38, 553–554 (2022).
Google Scholar
Chaikof, E. L. et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 67, 2–77.e2 (2018).
Google Scholar
Howard, D. P. J. et al. Population‐based study of incidence of acute abdominal aortic aneurysms with projected impact of screening strategy. J. Am. Heart Assoc. 4, e001926 (2015).
Google Scholar
Dahl, M., Liisberg, M., Stenehjem, M., Al Obeidi, I. & Sanddal Lindholt, J. Screening for abdominal aortic aneurysms still prevents ruptures: a secondary analysis of the VIVA trial. J. Am. Coll. Cardiol. 84, 2494–2496 (2024).
Google Scholar
Anjorin, A. C. et al. Underutilization of guideline-based abdominal aortic aneurysm screening in an Academic Health System. Ann. Vasc. Surg. 83, 184–194 (2022).
Google Scholar
Won, D. et al. Sound the alarm: the sonographer shortage is echoing across healthcare. J. Ultrasound Med. 43, 1289–1301 (2024).
Google Scholar
Chiu, I.-M. et al. Prospective clinical evaluation of deep learning for ultrasonographic screening of abdominal aortic aneurysms. npj Digit. Med. 7, 1–6 (2024).
Viz.ai | AI-Powered Care Coordination. Viz.ai, the Proven AI-Powered Care Coordination Platform https://www.viz.ai/.
How Viz.ai hit $48.8M revenue with a 325 person team in 2024. Latka https://getlatka.com/companies/viz.ai.
Publications Archive. Viz.ai, the Proven AI-Powered Care Coordination Platform https://www.viz.ai/publications.
Pirahanchi, Y. et al. PFO‐ACCESS: Augmenting Communications for Medical Care or Closure in the Evaluation of Patients With Stroke With Cardiac Shunts. Stroke.: Vasc. Interv. Neurol. 5, e001707 (2025).
Colasurdo, M. et al. Estimation of ventricular and intracranial hemorrhage volumes and midline shift on an external validation data set using a convolutional neural network algorithm. Neurosurgery (2022).
Figurelle, M. E. et al. Viz.ai implementation of stroke augmented intelligence and communications platform to improve indicators and outcomes for a comprehensive stroke center and network. AJNR Am. J. Neuroradiol. 44, 47–53 (2023).
Google Scholar
Karamchandani, R. R. et al. Automated detection of intracranial large vessel occlusions using Viz.ai software: Experience in a large, integrated stroke network. Brain Behav. 13, e2808 (2023).
Google Scholar
Viz.ai Strengthens Information Security with ISO-27001:2022 Certification. Viz.ai, the Proven AI-Powered Care Coordination Platform https://www.viz.ai/news/viz-ai-strengthens-information-security-with-iso-certification.
van Leeuwen, K. G. et al. Cost-effectiveness of artificial intelligence aided vessel occlusion detection in acute stroke: an early health technology assessment. Insights Imaging 12, 133 (2021).
Google Scholar
Viz.ai revenue, valuation & growth rate. https://sacra.com/c/viz-ai/.
FDA approves stroke-detecting AI software. Nat. Biotechnol. 36, 290–290 (2018).
Hassan, A. E. New technology add-on payment (NTAP) for Viz LVO: a win for stroke care. J. Neurointerv. Surg. 13, 406–408 (2021).
Google Scholar
Chen, M. M., Golding, L. P. & Nicola, G. N. Who will pay for AI? Radiol. Artif. Intell. 3, e210030 (2021).
Google Scholar
Parikh, R. B. & Helmchen, L. A. Paying for artificial intelligence in medicine. NPJ Digit. Med. 5, 63 (2022).
Google Scholar
Abràmoff, M. D. et al. A reimbursement framework for artificial intelligence in healthcare. NPJ Digit. Med. 5, 72 (2022).
Google Scholar
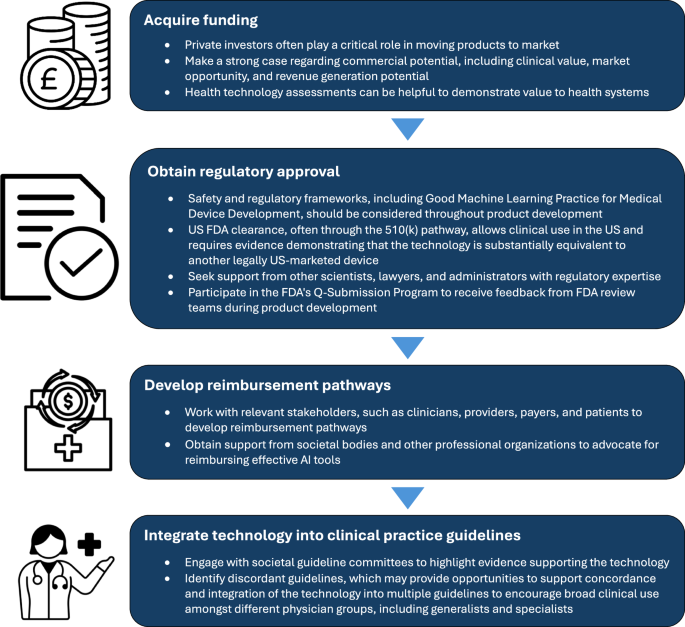
US Food & Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. (2025).
Muralidharan, V. et al. A scoping review of reporting gaps in FDA-approved AI medical devices. npj Digit. Med. 7, 1–9 (2024).
Wu, K. et al. Characterizing the clinical adoption of medical AI devices through U.S. Insurance Claims. NEJM AI 1, AIoa2300030 (2024).
Thomas, R. & Chalkidou, K. Cost–effectiveness analysis. In Health system efficiency: How to make measurement matter for policy and management [Internet] (European Observatory on Health Systems and Policies, 2016).
Li, X., Zhao, G., Zhang, J., Duan, Z. & Xin, S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population–a meta-analysis. PLoS ONE 8, e81260 (2013).
Google Scholar
Monaco, L. et al. The complexity of funding rare disease research: an IRDiRC assessment of the landscape. rdodj 3, 27 (2024).
Vervoort, D. et al. One-time screening for abdominal aortic aneurysm in Ontario, Canada: a model-based cost-utility analysis. Can. Med. Assoc. J. 196, E112–E120 (2024).
National Academies of Sciences, E., Division, H. and M., Health, B. on G. & States, C. on G. H. and the F. of the U. Promoting Cardiovascular Health and Preventing Cancer. in Global Health and the Future Role of the United States (National Academies Press (US), 2017).
Nishimiya, K., Matsumoto, Y. & Shimokawa, H. Recent advances in vascular imaging. Arterioscler. Thromb. Vasc. Biol. 40, e313–e321 (2020).
Google Scholar
Trowman, R., Migliore, A. & Ollendorf, D. A. The value and impact of health technology assessment: discussions and recommendations from the 2023 Health Technology Assessment International Global Policy Forum. Int. J. Technol. Assess. Health Care 39, e75 (2023).
Google Scholar
US Food & Drug Administration. Good Machine Learning Practice for Medical Device Development: Guiding Principles (2025).
Lottes, A. E. et al. Navigating the regulatory pathway for medical devices—a conversation with the FDA, clinicians, researchers, and industry experts. J. Cardiovasc. Transl. Res. 15, 927–943 (2022).
Google Scholar
Office of the Commissioner (2025) U.S. Food and Drug Administration. FDA https://www.fda.gov/.
Dhruva, S. S., Darrow, J. J., Kesselheim, A. S. & Redberg, R. F. Experts’ views on FDA regulatory standards for drug and high-risk medical devices: implications for patient care. J. Gen. Intern. Med. 37, 4176–4182 (2022).
Google Scholar
Jhawar, N. et al. Impact of professional society guideline publications in medicine subspecialties from 2012 to 2022: implications for clinical care and health policy. Mayo Clin. Proc. Innov. Qual. Outcomes 7, 262–266 (2023).
Google Scholar
US Preventive Services Task Force. et al. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. J. Am. Med. Assoc. 322, 2211–2218 (2019).
Ahmed, M. I. et al. A systematic review of the barriers to the implementation of artificial intelligence in healthcare. Cureus 15, e46454 (2023).
Google Scholar
link






